
María de la Paz Fernández, who joined the College as assistant professor of neuroscience and behavior in 2019, recently published a study in Current Biology on circadian clocks that shows some segments of the circadian neurons don’t exclusively serve output functions, as originally thought, but also act as receivers. With this knowledge, scientists can better address circadian rhythm disruptions in humans, which, if left unchecked, can lead to inflammation and contribute to health problems related to blood pressure and cardiovascular function, as well as mood disorders.
The research was conducted at Barnard and published in collaboration with researchers from the Shafer Lab at the Advanced Science Research Center at the Graduate Center, CUNY. One of the authors is Fernández’s first Barnard student, Ausra Pranevicius ’22.
Working alongside students at her Neurobiology Lab on campus, Fernández’s research teams use Drosophila melanogaster (fruit flies) as a model to study underlying aggressive behavior and territoriality, as well as the connections between the circadian clock and social behaviors. As a Summer Research Institute (SRI) faculty mentor, Fernández incorporates real brains from humans and other species into her “Introduction to Neuroscience” course on campus to provide students with literal hands-on experience to connect what they’ve learned in the classroom with what they’re holding in their hands in the lab. (View the video below.)
For this Break This Down Q&A, Fernández discusses the surprise results behind the first paper from her lab at Barnard and what it meant to publish that paper with a student.
How do circadian network neurons function?
The circadian clock neuron network is the Drosophila melanogaster equivalent of the mammalian suprachiasmatic nuclei, or SCN, which is the timekeeping center of the brain. These neurons are organized in several different clusters that play different roles in behavior. For example, some are more active in the morning, and some are more active in the evening. The daily wake/activity cycles that we see in all animals are the result of the combined action of those different groups of neurons.
For an animal to exhibit a normal behavior under light and dark cycles, those different clusters of neurons must be capable of acting in synchrony. If some of those neurons are disconnected from the other groups, we can see the effect on behavior. It is similar to having an orchestra in which some members are no longer following the conductor.
What did your research team learn about the dorsal medial termini and how it affects the circadian network and rhythm?
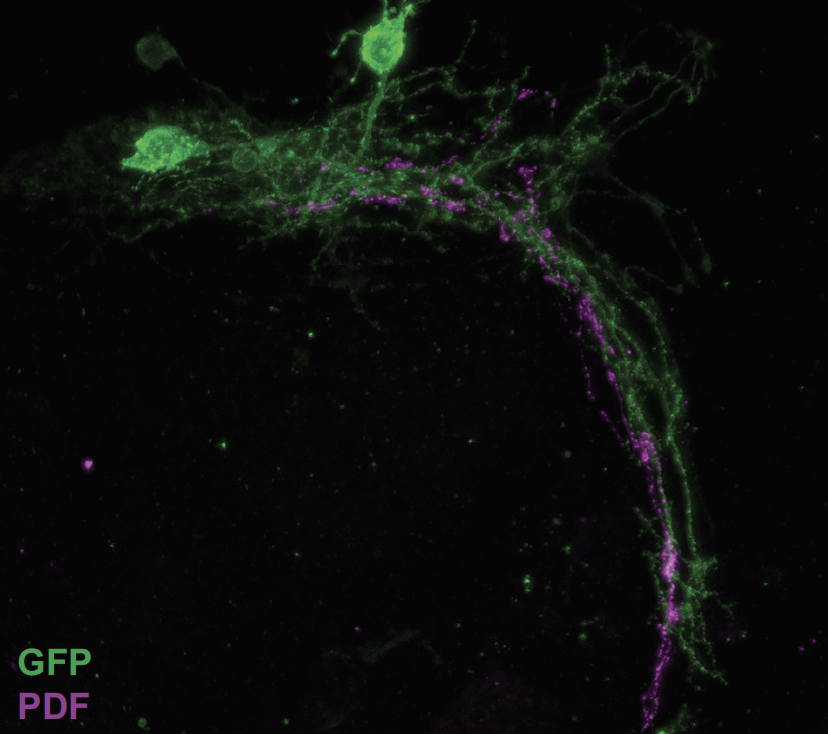
We focused on one group of clock neurons in particular, the small lateral ventral neurons [s-LNVs]. These cells are considered the pacemaker, because they play a key role in keeping the other neurons in the network synchronized. We studied the microanatomy of these neurons to understand how they are connected with other cells.
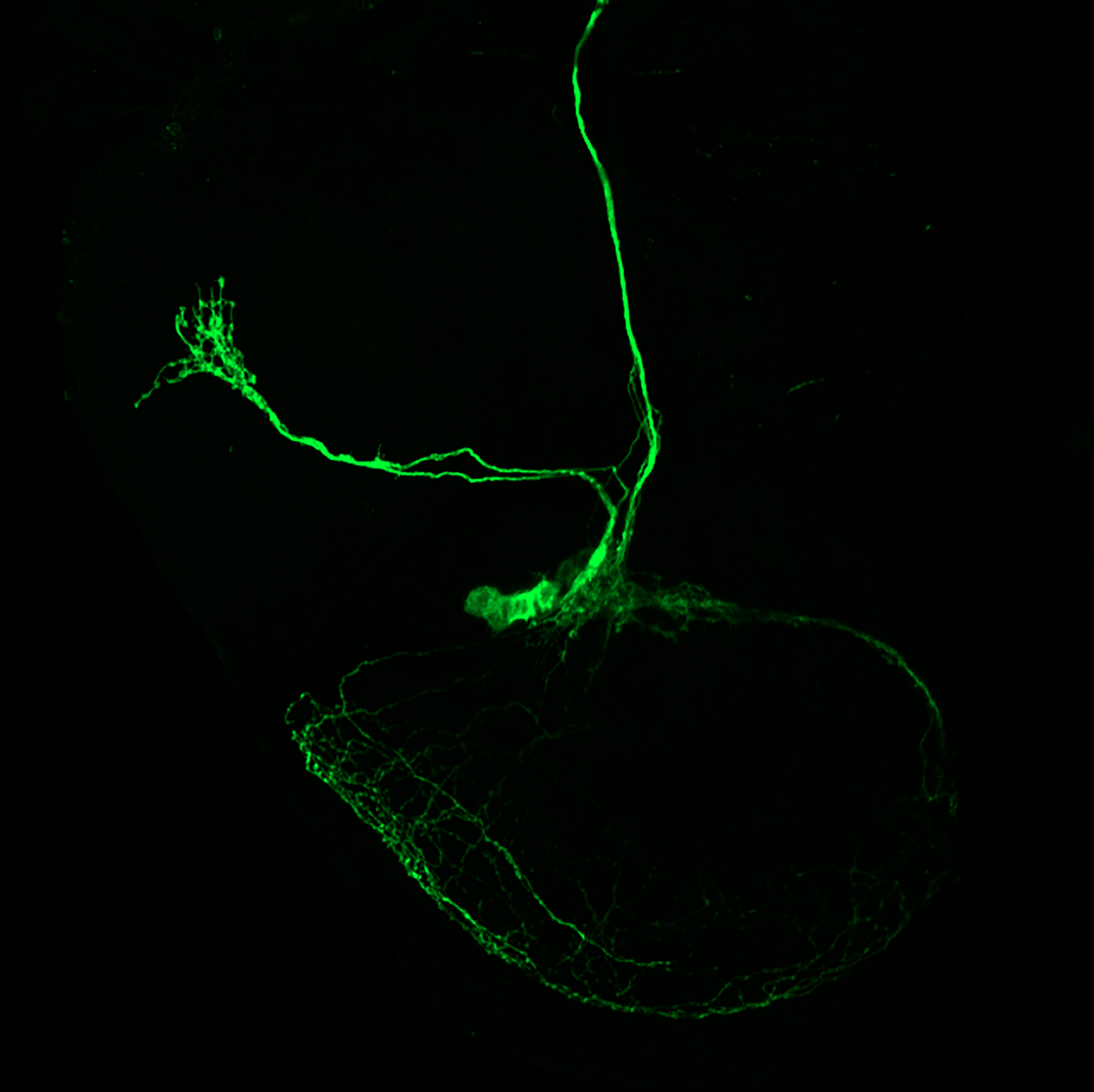
We know that these neurons, the s-LNVs, undergo structural plasticity, changing their morphology between night and day. This is something I discovered a long time ago, when I was a graduate student; we further explored and built upon this finding in our recent study. Their dorsal termini, which is the site where their projections end, is more complex in the morning than at night, and this rhythm in morphology persists even in the absence of environmental conditions. In this paper, we showed that the dorsal termini of the s-LNVs play a key role in receiving information from other neuronal clusters in the clock network.
Why were the results surprising?
Due to several interesting observations from different laboratories about their function and relative position of these dorsal termini of the s-LNVs, many people believed that these changes in microanatomy, which we call structural plasticity, were mainly a mechanism that allowed these clock neurons to communicate with their downstream targets — an output pathway.
Here, we showed that in the absence of these termini, the classic behavioral outputs don’t seem to be affected. However, there appear to be major consequences in terms of receiving information from other clock cells, the dorsal neurons, that are necessary to respond to temperature changes in the environment. This indicates that the dorsal termini play a role in the input pathways to the s-LNVs.
This work was performed in conjunction with the Shafer Lab at the Advanced Science Research Center (ASRC) and is the result of several years of collaboration. It is the first paper from my lab at Barnard, and my first Barnard student, Ausra Pranevicius ’22, is a co-author on this article. Ausra was working with me before I started my lab at Barnard, helped me move it to Barnard, and even helped to train other students. And she is actually an artist [@aurandomness]. Ausra’s excellent drawings are displayed all over the lab. I think the attention to detail that makes her so talented at drawing also helps in her research; she often “sees” things that nobody else sees. The quality of her data is impressive. I am really proud of her.
How do your study’s findings help us better understand how the environment influences circadian rhythms and other metabolic processes in humans?
These findings help us understand the mechanisms by which different components of the circadian clock neuronal network are connected to each other. An interesting implication of this study is the role that nonsynaptic transmission plays in connectivity within the circadian network. In the absence of the termini, the behavioral outputs seem unaffected, which suggests that the s-LNVs are still able to communicate with their downstream targets. One of the possibilities is that the neuropeptide that they release — pigment-dispersing factor (PDF) — is still being released in a way that allows it to reach the right targets at the right time.
The circadian clock influences multiple aspects of physiology and behavior, from gene expression to metabolism to sleep-wake cycles. Learning the mechanisms that allow different components of the circadian clock network to be connected helps us understand how these neurons can operate in synchrony to control processes that are key for our physical and mental health.
What health risks do we face when our circadian rhythms fall out of alignment with cues in the environment?
The circadian clock is believed to have evolved as a mechanism that allowed organisms to adjust to the environmental changes that occured daily as a consequence of the Earth’s rotation. Different species have evolved to have their activity consolidated at different times of the day, and being ready to find resources, such as food or a potential mate, represents an advantage.
From a health perspective, in recent years, there has been an increasing number of studies showing that circadian misalignment, in which individuals are not synchronized to their environment, has negative effects on blood pressure, inflammation, cardiovascular function, and mood disorders. The most classic case of misalignment in humans is that of shift workers, and the adverse health consequences of forcing our bodies to adhere to such schedules is well established.
To see how Professor Fernández uses real brains to teach students about about the nervous system, watch the video below:
For tips on how to get a better night’s sleep, read President Sian Leah Beilock’s recent Forbes piece on why sleep is especially important during the pandemic and this article in Women's Health about how to boost your immune function (spoiler: adequate shuteye is key).
Barnard experts explain.



